Serveis
Serveis de Bioanàlisi i CMC
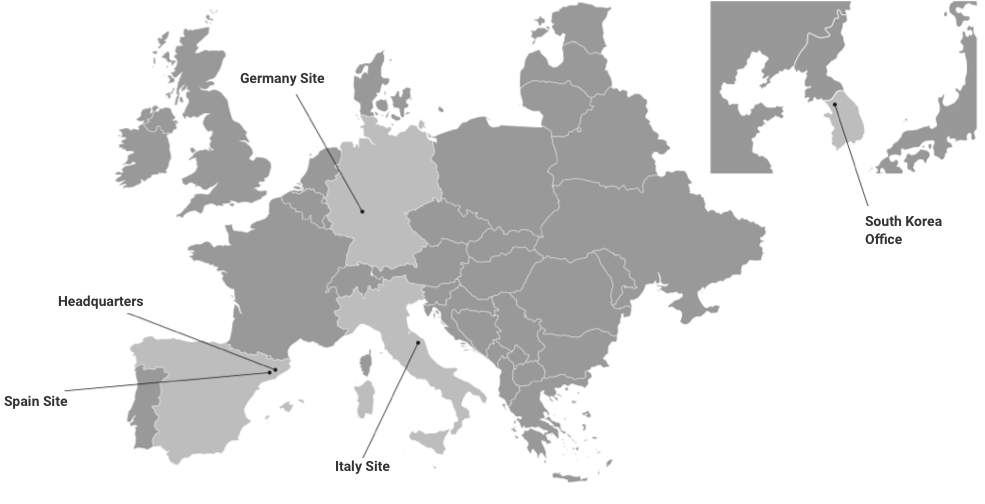
Kymos Group, amb 3 laboratoris a Espanya, Itàlia i Alemanya, es dedica a proporcionar solucions de bioanàlisi o anàlisi CMC durant tot el cicle de vida del producte farmacèutic, des de la investigació i el desenvolupament inicial fins a la fabricació i la comercialització.
Molècules
Serveis CRO basats en les especificacions de les molècules
Som experts en l’anàlisi de molècules petites, biològics, TIDES i teràpies avançades.
Sobre Kymos
Serveis CRO: certificats per GLP i GMP, inspeccionats per l’EMA i la FDA
El Grup Kymos és una CRO analítica amb tres laboratoris europeus dedicada a proporcionar serveis bioanalítics i CMC per a la indústria de les ciències de la vida durant tot el cicle de vida del producte: des de la recerca i el desenvolupament inicials fins a la fabricació i la comercialització. Tenim la certificació GLP i GMP, hem estat inspeccionats amb èxit per l’EMA, l’ANVISA i la FDA i som experts en treballs analítics i de control de qualitat amb molècules petites, biològics, TIDES (oligonucleòtids i pèptids) i teràpies avançades. El Grup Kymos ofereix serveis a clients de tot el món en les indústries farmacèutica, biotecnològica, veterinària, química fina, cosmètica i nutracèutica.
Bloc
Últimes notícies i informació
Amb més de 24 anys d’experiència, Kymos comparteix informació clau, estratègies i tendències del sector. Explora els nostres últims blocs:
Poseu-vos en contacte amb nosaltres