Services
Bioanalysis and CMC Services
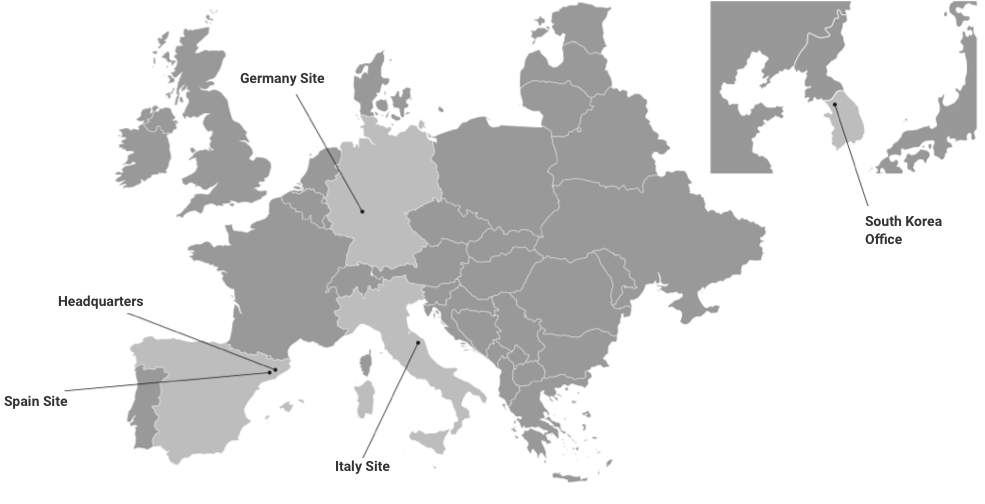
Kymos Group with 3 laboratories in Spain, Italy and Germany is devoted to providing any bioanalysis or CMC testing solution that you may need during the whole life cycle of your product, from early research and development to manufacturing and commercialization.
Molecules
CRO Services based on molecule specifications
We are experts in the analysis of small molecules, biologics, TIDES, and advanced therapies.
About Kymos
CRO Services: GLP- and GMP-certified, EMA and FDA inspected
The Kymos Group is an analytical CRO with three European laboratories devoted to providing bioanalytical and CMC services for the life science industry throughout the entire product life cycle: from early research and development to manufacturing and commercialization. We are GLP- and GMP-certified, have been successfully inspected by the EMA, ANVISA and the FDA and are experts in analytical and quality control work with small molecules, biologics, TIDES (oligonucleotides and peptides) and advanced therapies. Kymos Group provides services to clients worldwide in the pharmaceutical, biotechnology, veterinary, fine chemistry, cosmetic and nutraceutical industries
Blog
Latest news & insights
With over 24 years of experience, Kymos shares key insights, strategies, and industry trends. Explore our latest blogs:
Get in touch with us