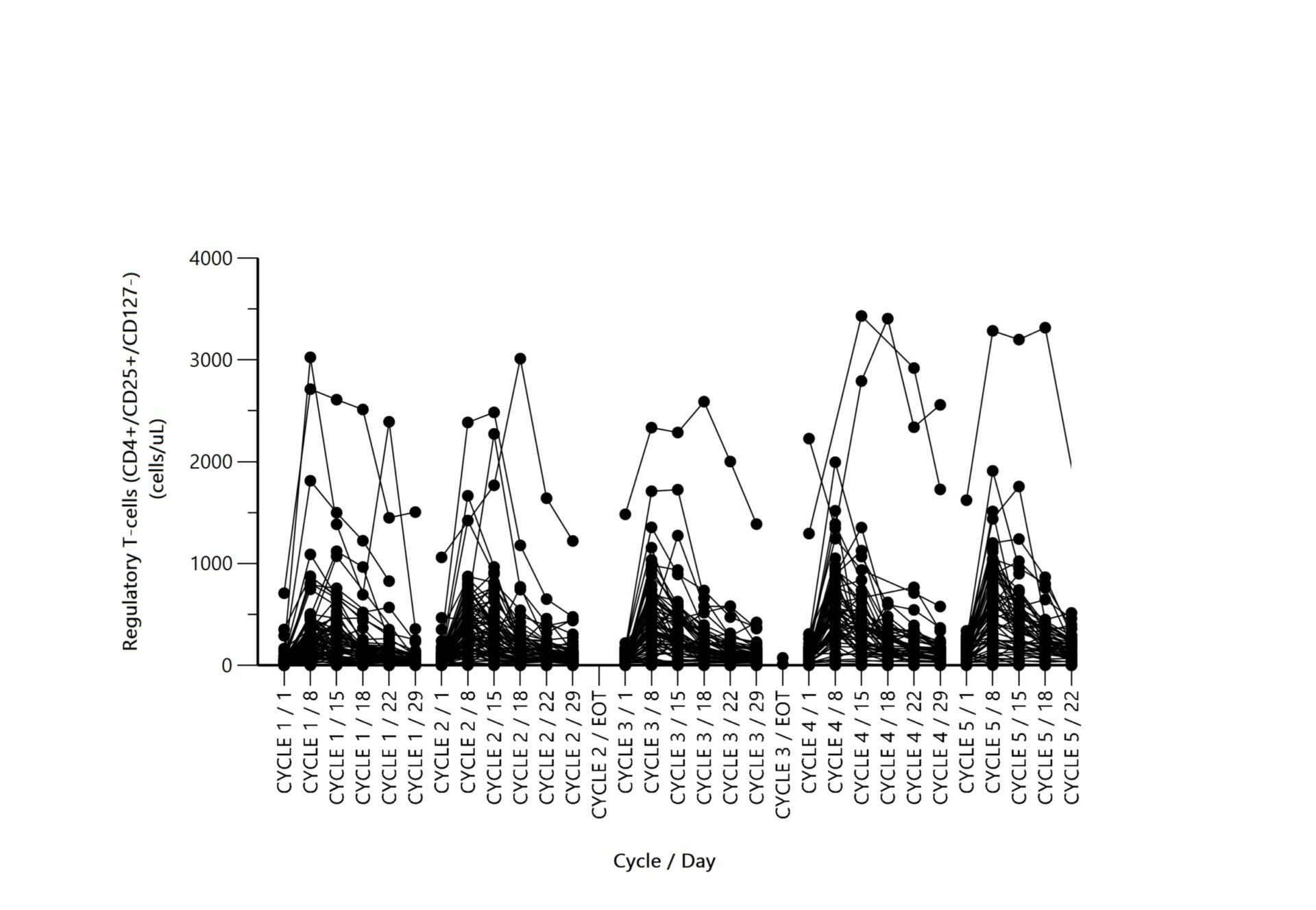
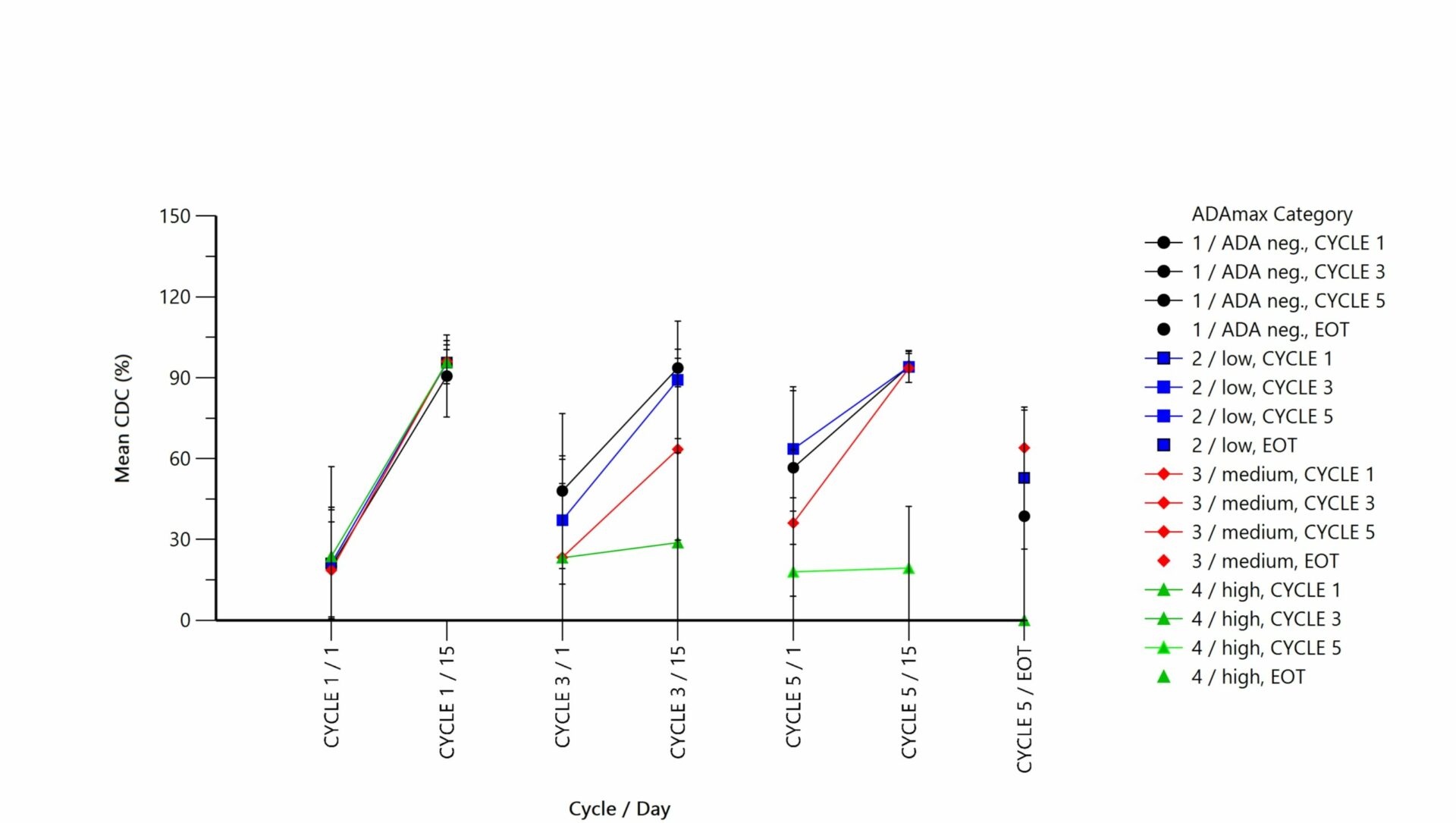
We have decades of experience providing pharmacokinetic evaluation and statistics, pharmacodynamics, and toxicokinetic calculations with Phoenix WinNonlin® for bioanalytical data coming from preclinical and clinical studies. These pharmacokinetic services are offered individually or as a packaged clinical study and include:
SOFTWARE
Our technology
Pharmacokinetic evaluation is conducted using the gold standard Phoenix WinNonlin® software 8.2 to:
- Analyze multiple matrices (plasma, serum, urine, blood, etc..), dosing schedules, and dosing routes using NCA.
- Perform compartmental modeling using built-in models or self-generated ones.
- Evaluate the bioequivalence of averages, individuals, or populations.
Services
Related services to Pharmacokinetic Evaluation & Statistics
Contact




