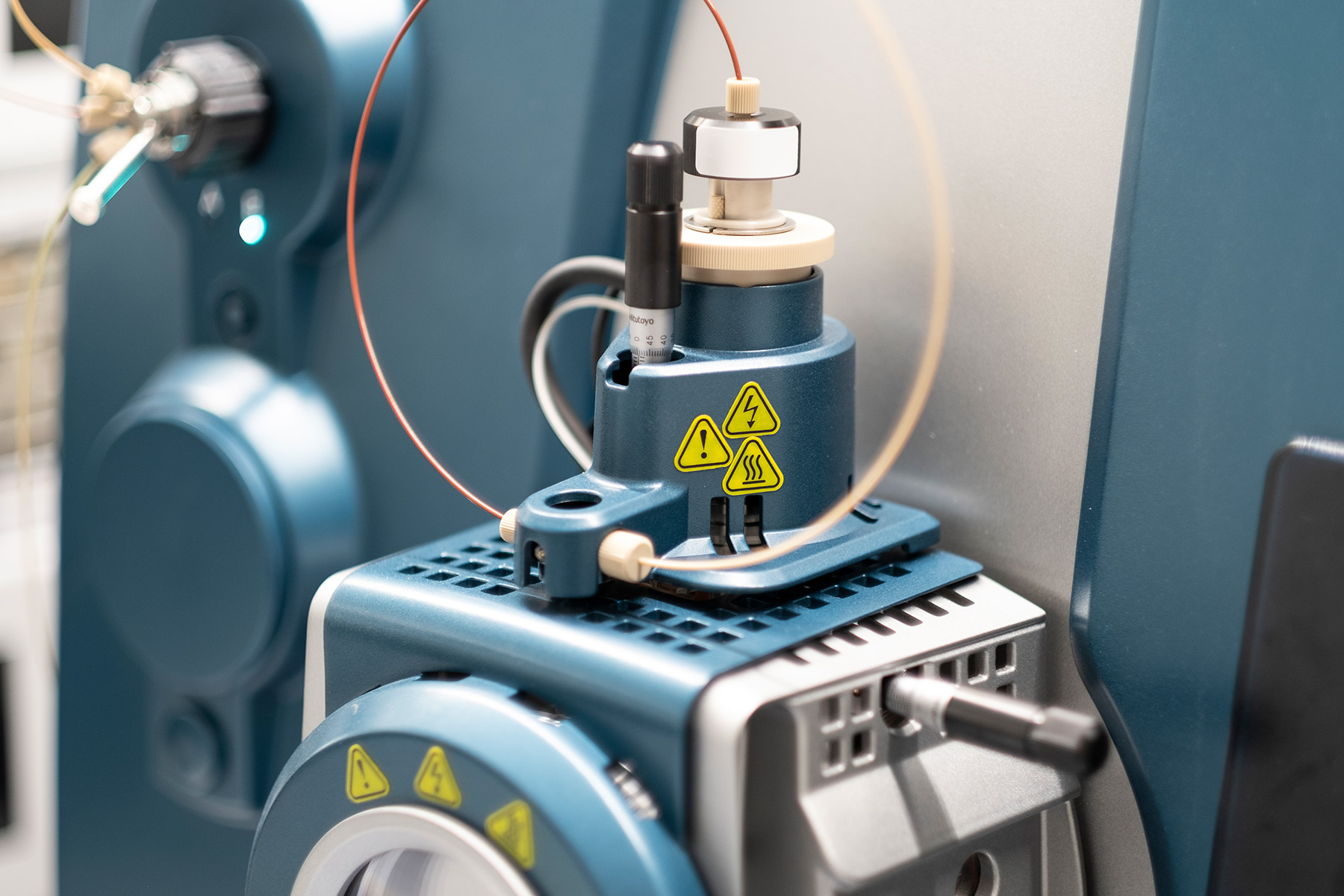
Serveis preclínics i clínics: conforme a GLP i GCP
Testimonials

“We are currently developing multiple new biosimilar projects and consider Kymos one of the best CROs in Europe for drug release and testing.”

“We were positively impressed when Kymos reacted to our needs by investing in their stability capabilities, and now, two years down the line, with projects in full-swing, I can safely say that we made the right choice. In Kymos we have found a reliable, committed, trustworthy and professional partner.”

“Back in 2017 there weren’t many analytical CROs able to carry out Franz cell assays and Kymos’ pioneering work was pivotal for the registration of our product.”

“Kymos offered superior historical expertise by being exposed to so many different clients, pharmacological compounds, and therapeutic areas. They addressed and anticipated very diverse problems.”

“I have had a long and fruitful experience with Kymos and have a huge interest in their participation owing to their reliability.”

“Prolytic is our analytical partner throughout the entire drug development process. Such a long and complex process requires a laboratory you can trust. Professional yet flexible, Prolytic accompanies us through the preclinical and clinical phases.”

“Establishing robust miRNA methods for valuable results is tricky. The team at Prolytic have been a key collaboration partner. With their 30 years of experience in bioanalytics and broad know-how in different techniques, study types, and substance groups, we were able to quickly develop a reproducible and reliable analysis method for miRNAs.”
BIO-Europe Spring: Milà, IT | 25-26 de març de 2025
German Pharm-Tox Summit: Hannover, DE | 25-28 de març de 2025
German Biotech Days: Heidelberg, DE | 09-10 d’abril de 2025
MEET2WIN: Bourdeaux, FR | 06-07 de maig de 2025
BIO KOREA 2025: Seül, KR | 07-09 de maig de 2025
BIONNALE 2025: Berlín, DE | 14-15 de maig de 2025
International Oligonucleotides and Peptides Conference: París, FR | 22 de maig de 2025
MabDesign Partnering Day: Lyon, FR | 02 de juny de 2025
Congrès Polepharma Biotesting: Evreux, FR | 04-05 de juny de 2025
International Oligonucleotides and Peptides Conference: Praga, CZ | 09-11 de juny de 2025
AFI Simposio: Rimini, IT | 11-13 de juny de 2025
Antibody Industrial Symposium (AIS): Tours, FR | 25-26 de juny de 2025