Sobre Kymos Group
Kymos Group és una organització europea de recerca per contracte (CRO) certificada per GLP/GMP, que compleix amb les normes GCP i està inspeccionada per l’EMA, l’ANVISA i la FDA, i que ofereix una gamma completa de serveis bioanalítics i de control de qualitat per a molècules petites, productes biològics i teràpies avançades.
El nucli del nostre ésser
La nostra visió i missió
Estem totalment compromesos amb tenir un impacte social promovent la diversitat, la igualtat de gènere, el benestar dels empleats, la protecció del medi ambient i la responsabilitat social.
La nostra visió és ser una CRO i CLO de classe mundial que ofereixi una cartera completa i innovadora de serveis de valor afegit que contribueixi a la comercialització de productes farmacèutics per al benefici final dels pacients.
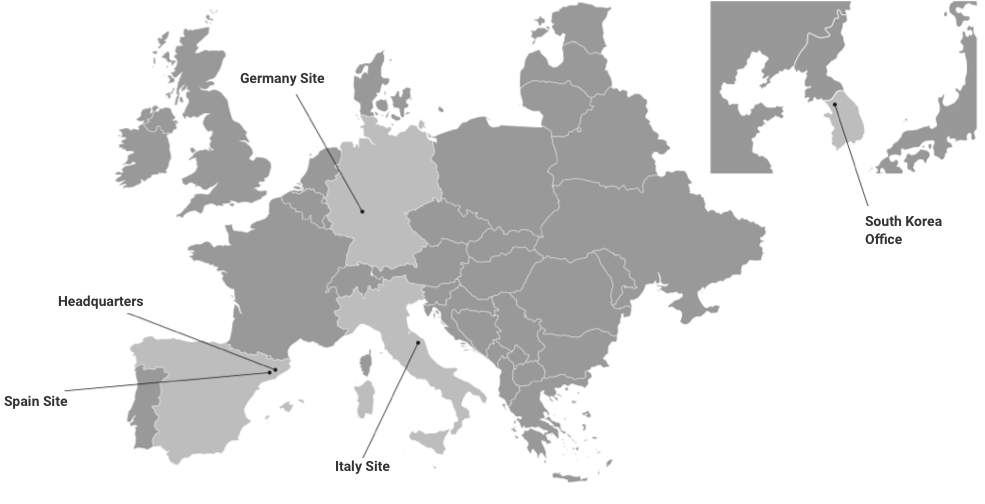
Tres laboratoris a Europa: un sol grup
El nostre abast global
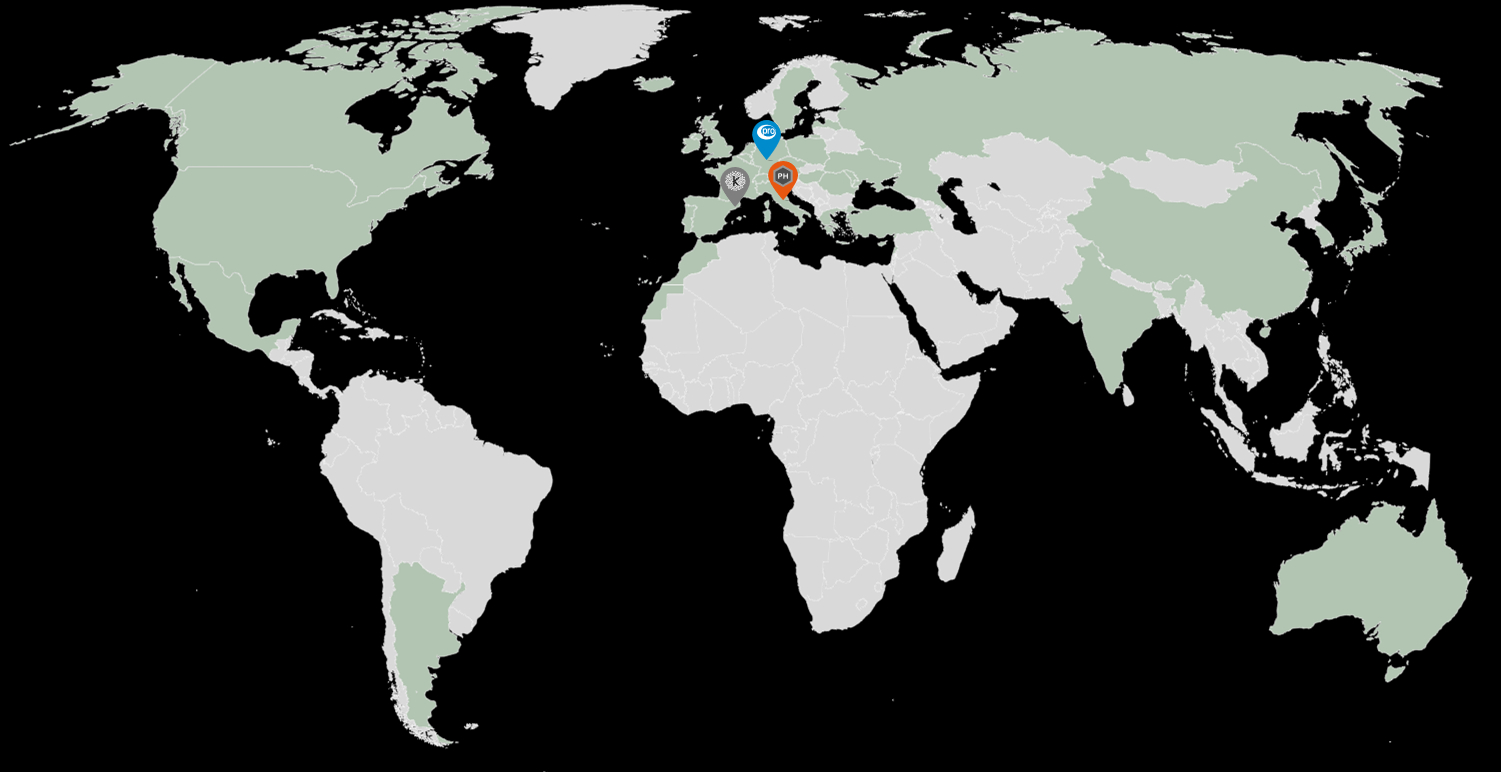
La nostra seu i el laboratori principal es troben a Barcelona (Kymos Espanya), i tenim laboratoris addicionals a Frankfurt (Kymos Alemanya, originalment Prolytic) i Ancona (Kymos Itàlia, originalment Pharmaprogress).
Kymos Group es compromet a donar suport a clients farmacèutics, biotecnològics, genèrics i biosimilars en més de 60 països d’arreu del món, ajudant-los a desenvolupar els seus productes, reduint costos i millorant el temps de comercialització amb el nostre enfocament únic i personalitzat i la nostra comprensió multidisciplinària de tot el procés de desenvolupament i fabricació: des de la R+D inicial, el desenvolupament del producte, les fases preclíniques i clíniques fins a l’aprovació final i els requisits posteriors a la comercialització .
Kymos Espanya
Kymos SL va ser fundada a Barcelona l’any 2001 per un equip de científics d’R+D amb dècades d’experiència en desenvolupament farmacèutic, des de la investigació inicial fins a la formulació final i els assaigs a gran escala per a la comercialització.
- Bioanàlisi de molècules petites (també conegut com Espec. de masses i permeació)
- Bioanàlisi de grans molècules (també conegut com Immunologia)
- Desenvolupament analític i validació
- Control de qualitat de petites molècules
- Control de qualitat de grans molècules (també conegut com proves biofarmacèutiques)
- Anàlisi elemental
- Microbiologia
- Estabilitats
Kymos, Alemanya
Prolytic GmbH va ser fundada l’any 2002 per un grup veterà de científics de Viatris GmbH, la unitat de negoci més gran de Degussa/ASTA Medica AG. El recentment constituït CRO, amb seu a Frankfurt, es va centrar en la prestació de serveis bioanalítics i de farmacocinètica als clients del sector farmacèutic. El 2020 Prolytic va passar a formar part del grup Kymos.
- Bioanàlisi de petites molècules (també coneguda com a cromatografia)
- Bioanàlisi de grans molècules (també coneguda com a biologia)
- Bioanàlisi d’àcids nucleics
Kymos, Itàlia
Pharmaprogress srl va ser fundada legalment l’any 1999 per l’antic executiu del grup Angelini i va començar a operar com a CRO l’any 2001. Es va construir un laboratori de 700 m2 adaptat a les seves necessitats a Camerata Picena, prop d’Ancona, i va ser aprovat per les autoritats sanitàries italianes segons les bones pràctiques de fabricació.
Més tard, el 2023, les instal·lacions es van traslladar a Monsano, on es va construir un nou laboratori de 1800 m2 que es va inaugurar l’any següent.
- Estabilitats, Desenvolupament Analític i Validació
- Control de qualitat de petites molècules (també conegut com a espectrometria de qualitat i masses)
Sucursal de Kymos a Corea del Sud
El 2024, Kymos Group va establir una oficina d’enllaç a Seül, Corea del Sud, per millorar el suport als nostres clients internacionals a la regió Àsia-Pacífic (APAC).
Situada a la prestigiosa Torre ASEM, aquesta oficina serveix com a punt de contacte principal per a les empreses farmacèutiques i biotecnològiques que operen a la regió.
SOBRE NOSALTRES
El nostre compromís i èxit
Més que un simple proveïdor de serveis, Kymos pretén ser un soci fiable: Totalment compromès amb els objectius dels nostres clients i amb els projectes que se’ns confien; oferir serveis d’alta qualitat, fiables en termes de preus per tal de mantenir la competitivitat dels nostres clients; mantenir els terminis perquè el temps de sortida al mercat és un element clau per a l’èxit; i constantment dedicat a la investigació i el desenvolupament com a única manera d’afrontar els nous reptes d’un entorn empresarial que canvia ràpidament.
Creixem constantment en percentatges de 2 dígits gràcies a la implantació de nous serveis i capacitats.
Estem orgullosos de tenir un percentatge molt alt de clients que tornen a l’any.
Disposem d’una elevada ràtio de cotitzacions aprovades, reflectint una oferta consistent en qualitat i preus justos.
El percentatge de nous clients augmenta any rere any, sobretot gràcies als nostres esforços en la internacionalització.
De la visió al creixement
La nostra història
Kymos SL va ser fundada l’any 2001 per un equip de científics d’R+D amb dècades d’experiència en desenvolupament farmacèutic, des de la investigació inicial fins a la formulació final i l’escalada dels assaigs per a la comercialització. L’empresa es va establir al Parc Científic de Barcelona i es va expandir ràpidament malgrat l’alta competència. El 2011, Kymos es va fer càrrec de l’equip d’immunologia d’Ipsen Pharma per crear una divisió biològica pionera. El 2015 Kymos es va traslladar a un nou laboratori al Parc Tecnològic del Vallès. El 2016 Kymos va adquirir CRO Pharmaprogress italià. El 2018, el creixement constant va requerir dues ampliacions successives amb un nou edifici d’oficines i laboratoris. El 2020 Kymos va adquirir el CRO Proytic alemany. El 2021, el grup Kymos està format per una seu de dos edificis més dos emplaçaments europeus, 14 departaments de laboratori, 170 experts altament formats i 5.000 m2 d’instal·lacions i instruments de primera línia. El 2023-2024 el nostre lloc italià es va ampliar amb un nou edifici i s’ha iniciat una ampliació del laboratori a Espanya.

Pharmaprogress va començar a prestar serveis a tercers com a CRO l’any 2001, tot i que la persona jurídica es va fundar anteriorment el 1999. A Camerata Picena, prop d’Ancona, es va construir un laboratori de 700 m2 apte per a l’ús i va ser homologat per GLP per les autoritats sanitàries italianes. La companyia va ser fundada per un antic gerent d’una empresa farmacèutica italiana.

Es va fundar Prolytic. Els orígens de Prolytic GmbH es remunten a Viatris GmbH & Co. KG, que forma part d’ASTA Medica AG, una empresa de recerca farmacèutica i anteriorment filial propietat al 100% de Degussa AG. Viatris GmbH & Co. KG va ser venuda a un inversor financer que va tancar les seccions de desenvolupament de fàrmacs “Bioanàlisi” i “Farmacocinètica”. Un grup experimentat de científics d’aquestes seccions va fundar Prolytic GmbH el desembre de 2002 amb l’objectiu d’oferir la seva abundant experiència en bioanàlisi i farmacocinètica als clients del sector farmacèutic.

Kymos va obtenir la certificació GLP l’any 2002, al principi. Els primers projectes van ser en metabolisme i bioanàlisi.

Prolytic va rebre la certificació GLP del Regierungspräsidium de Darmstadt en les categories 1 i 8.
Categoria 1: Propietats físiques i químiques i determinació del contingut
Categoria 8: Estudis analítics sobre materials biològics

Pharmaprogress va rebre l’aprovació GMP per a proves químiques i físiques de l’Agència Italiana de Medicaments (AIFA) el gener de 2009.
L’última autorització és la núm. aM-119/2016 del 22 de juliol de 2016, relativa als següents assaigs en medicaments d’ús humà:
1.6.3 Control de qualitat GMP, proves químiques i físiques
1.6.3 Control de qualitat GMP, proves químiques i físiques en productes medicinals en investigació

L’any 2009, l’empresa va obtenir l’autorització GMP i de fabricant (fabricant parcial per a activitats de control de qualitat). A partir d’aquest punt, Kymos va traslladar totes les activitats de CMC (principalment estudis de desenvolupament analític i d’estabilitat) al sistema de qualitat GMP i va començar a oferir serveis de control de qualitat per a API i DP.

El 2011, Kymos va construir un laboratori de microbiologia i va ampliar la seva certificació GMP a les proves microbiològiques de productes no estèrils. Aquesta certificació es va ampliar posteriorment per cobrir productes estèrils el 2016.

El gener de 2012, Kymos va arribar a un acord per fer-se càrrec del departament d’immunologia d’un antic centre d’R+D que el grup multinacional francès IPSEN tenia a Barcelona. Gràcies a aquest acord, Kymos va poder crear una nova divisió biològica. El personal experimentat, els instruments i el coneixement es van transferir a Kymos i ambdues empreses van signar un acord de col·laboració a llarg termini per desenvolupar medicaments innovadors conjuntament.

El gener de 2015, l’empresa va traslladar les seves instal·lacions de Barcelona (Parc Científic de Barcelona) a un edifici independent de Cerdanyola del Vallès (Parc Tecnològic del Vallès). Es va construir un nou laboratori que va augmentar la superfície de 650 m2 a 1.500 m2. Això significava que l’empresa podria augmentar la facturació i augmentar la seva plantilla el 2015 i el 2016. El mateix any, Kymos va obtenir l’autorització d’importació de productes medicinals. S’han implementat serveis de llançament per lots per a productes de màrqueting.

El novembre de 2016, l’empresa va adquirir una CRO a Itàlia anomenada Pharmaprogress. L’empresa estava situada a Ancona i tenia 8 persones a la plantilla. Aquesta empresa disposava d’uns 700 m2 de laboratoris de bioanàlisi i desenvolupament analític d’API i DP. En l’actualitat, l’empresa s’ha integrat plenament a Kymos i actualment compta amb 16 persones a la plantilla.

Al juliol, Kymos va ser sotmès a una inspecció general del lloc per part de la FDA. La inspecció es va completar amb èxit i es va emetre la carta EIR corresponent.

Al juliol, Kymos va ampliar les seves instal·lacions traslladant les oficines a un nou edifici independent proper a l’existent. Aquest nou edifici compta amb una sala de formació, un arxiu, diferents sales de reunions i una cafeteria.

A l’agost, Prolytic es va traslladar a una ubicació més gran i d’última generació a Weismüllerstraße 45. Doblant la mida, l’equip de 15 membres comptava ara amb 800 m² d’espai de laboratori i oficines per oferir la millor bioanalítica i farmacocinètica als seus clients.

L’abril, la FDA va acabar la inspecció del lloc de Pharmaprogrees a Ancona confirmant que aquesta instal·lació era apta per a sol·licituds de màrqueting als EUA. Ha estat una inspecció documental basada en l’anterior inspecció de l’AIFA (Agenzie Italiana del Farmaco) gràcies al nou acord de reconeixement mutu existent entre EMA i FDA.

Al maig, Kymos va tornar a ampliar les seves instal·lacions amb nous laboratoris de desenvolupament analític i proves biofarmacèutiques. Durant l’any, els serveis de proves per lots per a biosimilars i clients asiàtics estan impulsant el creixement de l’empresa.

L’octubre de 2020, Kymos es va fusionar amb el laboratori bioanalítica alemany Prolytic GmbH situat a Frankfurt. Prolytic tenia 16 persones a la plantilla i estava especialitzada en bioanàlisi de molècules petites i grans, així com en oligonucleòtids i farmacocinètica.

Kymos és una empresa molt internacional. A més de les tres seus en diferents països europeus, també treballem per a clients d’arreu del món. L’any 2021 hem superat per primera vegada el nombre de 40 països en què es troben els nostres clients que cobreixen tots els continents.
2022 Kymos va adquirir un edifici més ampli, còmode i d’última generació a la zona d’Ancona. Parts de l’edifici necessiten una renovació i encara ens queda molta feina per davant. Però l’any que ve, Pharmaprogress finalment es pot traslladar a les seves noves instal·lacions modernes.

Kymos va rebre la certificació oficial de l’ANVISA (Agència Nacional de Vigilància Sanitària) per als estudis de bioequivalència i se li va concedir permís per participar en aquests estudis per presentar-los al Brasil. Les obres del nou edifici de Pharmaprogress s’han finalitzat i hem començat a moure’s.

El nostre nou edifici (1800 m2) a Itàlia s’ha inaugurat oficialment a principis d’any, i tots els equips i projectes s’han traslladat a la nostra nova ubicació a Monsano. Han començat les obres de l’ampliació del laboratori a Espanya (+600 m²).

Get in touch with us