Pre-clinical & Clinical Bioanalysis · GLP & GCP Compliant
Comprehensive preclinical and clinical services designed to ensure GLP and GCP compliance.
Services
All Pre-clinical & Clinical Bioanalysis Services

Bioanalysis of Biologics and Biosimilars
Method development and validation and sample measurement of biologics and biosimilars.

Bioanalysis of Nucleic Acids
Quantification of nucleic acids as therapeutics, biomarkers or similar with standardized and reproducible methods according to GLP.

Bioanalysis of Small Molecules & Generics
Development and validation of analytical methods for analysis of biological samples delivered mainly from pre-clinical and clinical studies.

Bioequivalence Studies
Bioanalysis for bioequivalence studies of generics in collaboration with the sponsor and/or the selected clinical phase centre.

Percutaneous Absorption
Percutaneous absorption assays for topical semisolid drug products: In Vitro Release Test (IVRT) and In Vitro Permeation Test (IVPT)

Determination of Enzyme Activity
Determination of active enzymes and enzymatic biomarkers in biological and formulation samples according to GLP.

Biomarker Analysis
Biomarker analysis services across various therapeutic areas aided by our state-of-the-art equipment and multiplexing techniques.
Ready to accelerate your drug development?
Let’s tailor the perfect solution for you!
Ask our experts
Partnering with Kymos Group on our preclinical program for our first asset has been invaluable. Their team brought deep analytical expertise and ensured that biodistribution and histology assessments were conducted to the highest scientific and regulatory standards. Thanks to their responsiveness and commitment, we generated high-quality data that strengthened our preclinical package. We look forward to continuing our collaboration as we advance this innovative therapy into the clinic.
We were positively impressed when Kymos reacted to our needs by investing in their stability capabilities, and now, two years down the line, with projects in full-swing, I can safely say that we made the right choice. In Kymos we have found a reliable, committed, trustworthy and professional partner.
Get in touch with us
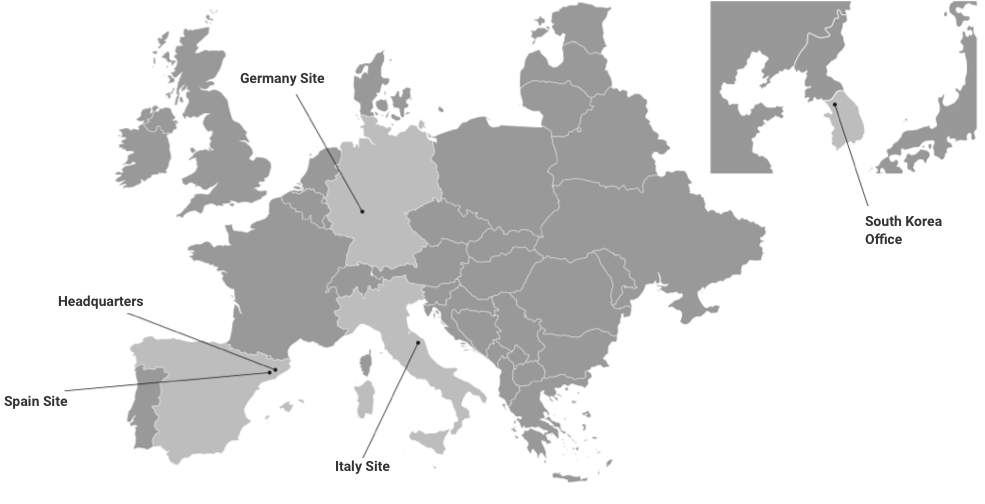
Find a location near you
SPAIN SITE
Ronda Can Fatjó 7B
08290 Cerdanyola del Vallès
Barcelona, Spain
VAT nº ES-B62170337
ITALY SITE
Via Emilia-Romagna, 28-30-32
60030 Monsano
Ancona, Italy P.
IVA 01564160420
GERMANY SITE
Weismüllerstraẞe 45
D-60314 Frankfurt/Main
Germany USt-IdNr:
DE 226546121
HEADQUARTERS
Ronda Can Fatjó 5D
08290 Cerdanyola del Vallès
Barcelona, Spain
VAT nº ES-B70843693