Kymos Group has a specialized and dedicated team focused on the development and validation of test methods for gene therapy medicinal products, specifically viral vectors such as Adeno-associated virus (AAV), Adenovirus (AdV), and Lentivirus (LV).
Our laboratories are GLP and GMP certified and inspected by both the EMA and FDA. We offer a complete quality control service in a regulated and validated environment, supporting you across all phases, from drug discovery to market approval, thus accelerating time-to-market for gene therapy products.
Equipped with state-of-the-art technologies and techniques to ensure the best quality and timeline optimization, such as qTOF-MS, SEC, and AEX HPLC, CE-LIF, icIEF, qPCR, and ELISA, we offer a comprehensive catalog of CMC pre-qualified assays for viral vectors. This combination of specialized scientists and advanced techniques allows us to adapt our implemented methods or develop new ones according to each study’s specific needs.
SERVICES
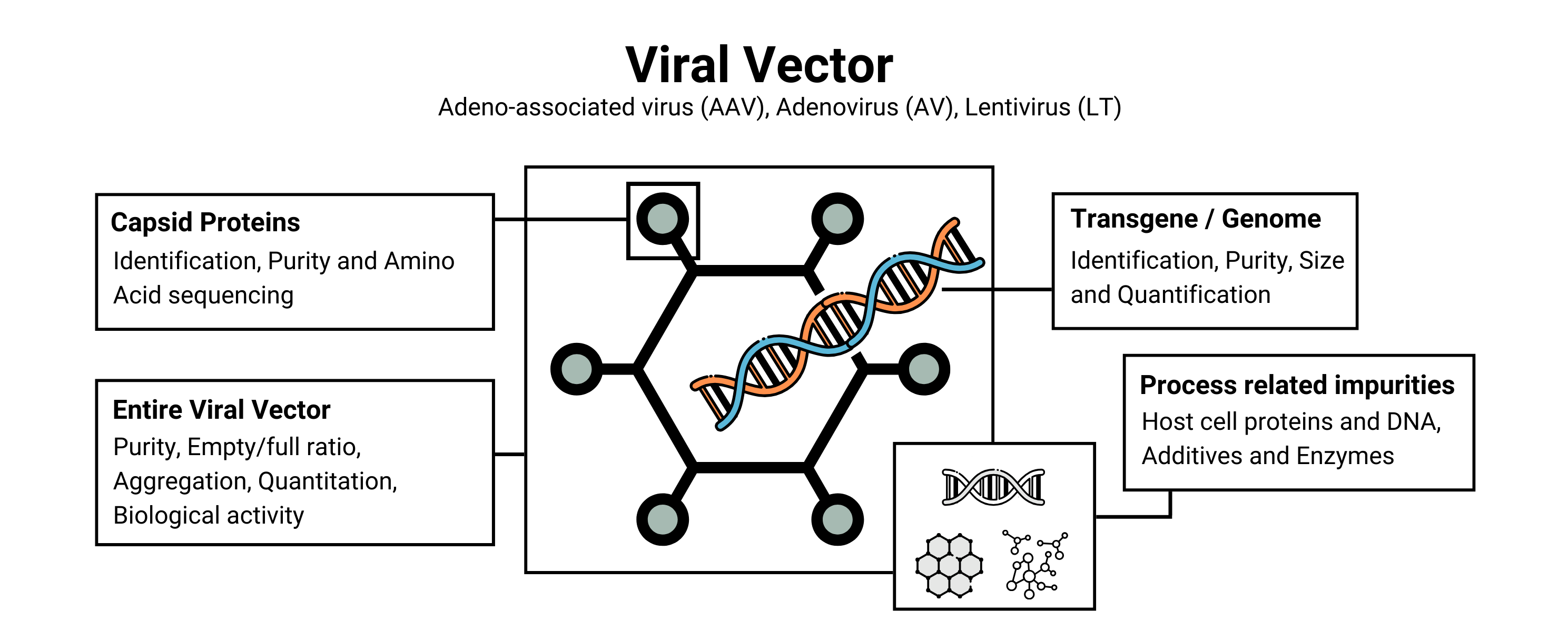
Viral Vector Analysis Services
Capsid Protein Characterization
Capsid of viral vectors are constituted by proteins. Their integrity and identity must be assessed using techniques used commonly for protein characterization. At Kymos Group are available the following assays:
- Molecular weight determination of protein constituents by Liquid chromatography coupled to High Resolution mass spectrometry (LC-HRMS)
- Correct ratio of protein constituents of the capsid and purity by Gel Capillary Electrophoresis (GCE)
- Correct ratio of protein constituents of the capsid and purity by SDS-PAGE
- Amino acid sequencing of the capsid proteins by enzyme digestion and LC-MS/MS analysis
Transgene/Genome Characterization
The transgene or genome of the viral vector that contains the information that must be transferred to the target cells has to be conveniently characterized and its purity and identity established. The following assays are developed by Kymos:
- Size identity and purity by Gel Capillary Electrophoresis with Laser Induced Fluorescence (GCE-LIF)
- Transgene Nucleotide Sequencing and DNA impurities by NGS (under development with a partner)
- Transgene quantitation by qPCR (under development)
- Total DNA quantitation by fluorescence methods
Entire Viral Vector Characterization
The quality attributes of the viral vector must be determined to assure the safety and efficacy of each manufactured batch. Several quality attributes can be tested at Kymos:
- Quantitation, empty/full capsids ratio, and purity by anion exchange chromatography (AEX)
- Empty/full capsids and aggregation by Transmission Electron Microscopy (TEM) (under development with a partner)
- Aggregation by Size Exclusion Chromatography (SEC)
- Quantitation by ELISA
- Biological activity, Protein expression
Process Related Impurities Analysis
Process-related impurities can have very different chemical structures: nucleic acids, proteins, small molecules, etc. Kymos has a wide range of analytical tools for the analysis of this wide range of compounds. Some examples are:
- Residual host cell proteins by ELISA
- Residual host cell and plasmid DNA by qPCR (under development)
- Residual host cell DNA size for lentivirus by Gel Capillary
- Electrophoresis with Laser Induced Fluorescence (GCE-LIF)
- Residual enzymes like benzonase by ELISA
- Residual antibiotics by HPLC

Why Choose KYMOS?
Services
Related services to Viral Vectors Characterization
Contact




