CMC Analysis & Quality Control ·
GMP-certified and EMA & FDA inspected
Reliable solutions for CMC and quality control, delivered by GMP-certified and FDA & EMA & ANVISA inspected labs.
Services
All CMC Services & Quality Control Services

Quality Control of Biologics and Biosimilars
Complex characterization projects for innovative biologics and comparison of biosimilars.

Quality Control of Oligonucleotides
Comprehensive CMC testing for synthetic oligonucleotides under GMP conditions.

Quality Control of Small Molecules
Analytical tests for APIs, excipients, intermediate products, finished products, packaging materials and process environment samples.

Viral Vector Characterization
GMP-certified characterization and definition of quality attributes of Adeno-associated virus (AAV), Adenovirus (AV), and Lentivirus (LT).

Method Development, Validation and Transfer
Development and validation of analytical methods for excipients, APIs, intermediate products and finished products.

Stability Studies
Full ICH studies and on-going stability programmes at different storage conditions.

Batch Testing and Batch Release
Our laboratories are GMP certified and we have a partial manufacturer authorization for Quality Control purposes.

Biosimilar Comparability Studies
Testing lab for biosimilar products – GMP inspected by U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA).

Extractables and Leachables
Semi-quantitative screening for both volatile, semi-volatile and non-volatile organic compounds.

Elemental, Nitrosamines & Other Impurity Testing
We offer GLP Cell-based neutralizing antibody assays for biologics and GMP Cell-based potency assays for vaccines and other biologics.

Microbiology
Laboratory including cleanroom with airlock technology and HEPA filters for testing of sterile and non-sterile APIs and drug products.

Cell-Based and Potency Assays
We offer GLP Cell-based neutralizing antibody assays for biologics and GMP Cell-based potency assays for vaccines and other biologics.
Ready to accelerate your drug development?
Let’s tailor the perfect solution for you!
Ask our experts
Partnering with Kymos Group on our preclinical program for our first asset has been invaluable. Their team brought deep analytical expertise and ensured that biodistribution and histology assessments were conducted to the highest scientific and regulatory standards. Thanks to their responsiveness and commitment, we generated high-quality data that strengthened our preclinical package. We look forward to continuing our collaboration as we advance this innovative therapy into the clinic.
We were positively impressed when Kymos reacted to our needs by investing in their stability capabilities, and now, two years down the line, with projects in full-swing, I can safely say that we made the right choice. In Kymos we have found a reliable, committed, trustworthy and professional partner.
Get in touch with us
Find a location near you
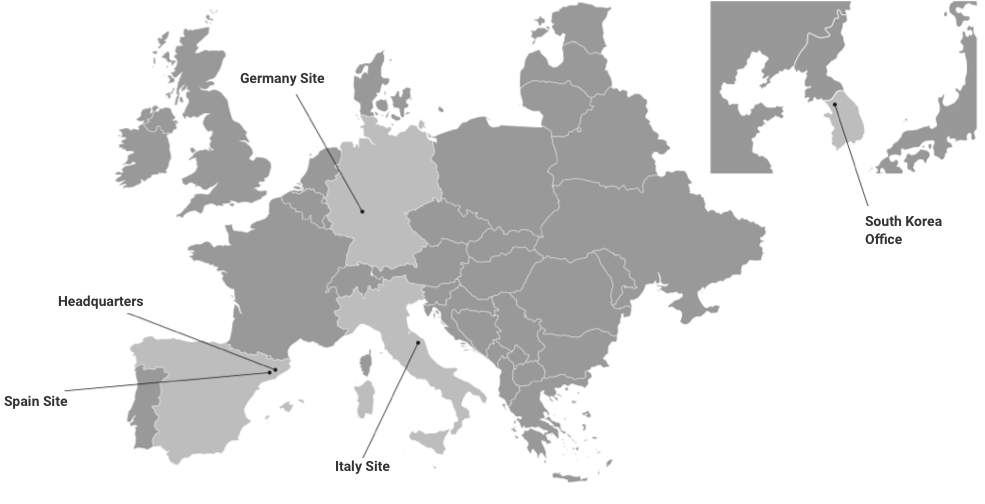
SPAIN SITE
Ronda Can Fatjó 7B
08290 Cerdanyola del Vallès
Barcelona, Spain
VAT nº ES-B62170337
ITALY SITE
Via Emilia-Romagna, 28-30-32
60030 Monsano
Ancona, Italy P.
IVA 01564160420
GERMANY SITE
Weismüllerstraẞe 45
D-60314 Frankfurt/Main
Germany USt-IdNr:
DE 226546121
HEADQUARTERS
Ronda Can Fatjó 5D
08290 Cerdanyola del Vallès
Barcelona, Spain
VAT nº ES-B70843693