Biologics and biosimilars are transforming modern medicine, but their complex structures demand rigorous analytical and regulatory support. At Kymos, we specialize in tailored bioanalysis and CMC services for biologics and biosimilars, helping pharmaceutical and biotech companies navigate development and commercialization with speed, precision, and compliance.
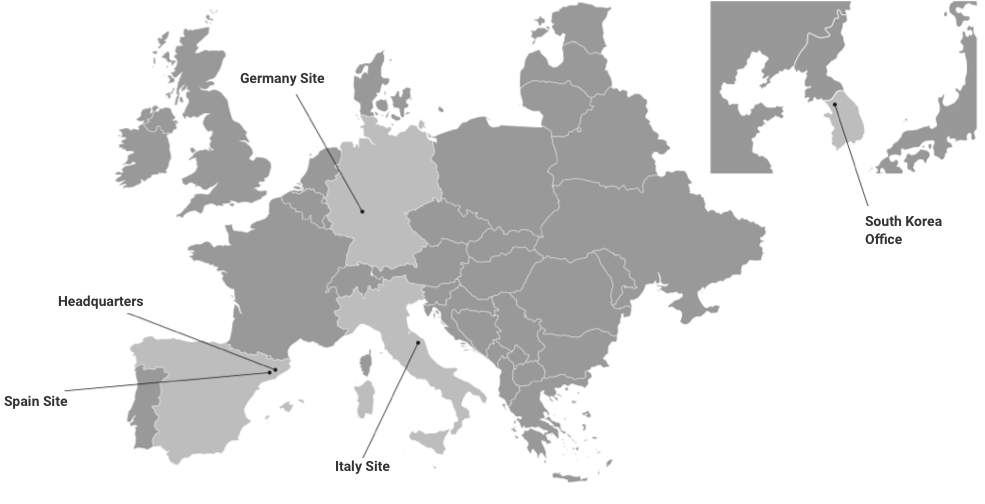
Our experienced scientific teams and specialized facilities, operating under GMP and GLP compliance and successfully inspected by the EMA and FDA, deliver reliable results that keep your biologics programs moving forward.
CMC & Quality Control:
- Physical-chemical parameters
- Potency assays (bioassays)
- Purity and aggregation
- Impurities (related substances, residual solvents, nitrosamines, elemental impurities)
- Protein interaction and protein binding
- Characterization studies
- Microbiological control (pathogen identification, sterility, endotoxins)
- Stability studies
- Comparability studies for biosimilars
- Batch testing and batch release

Why partner with Kymos for your Biologics?
Services
Our Biologics and Biosimilars Portfolio
Get in touch with us