Bioanalysis and CMC Services
Offering comprehensive bioanalysis and CMC testing solutions at every stage of your product’s research, development, and quality control.
Molecules
CRO Services based on molecule specifications
We are experts in the analysis of small molecules, biologics, TIDES, and advanced therapies.
Services
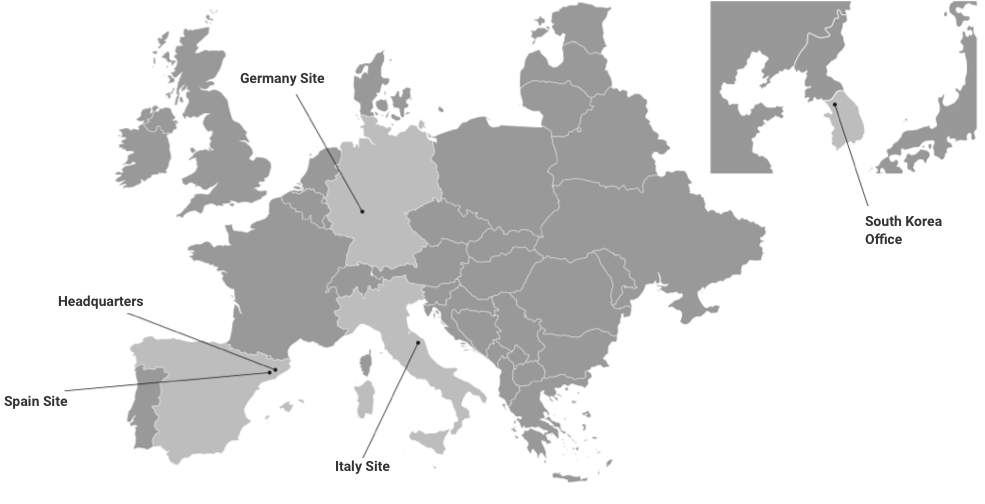
GMP and GLP-certified. FDA & EMA & ANVISA inspected labs.
With over 20 years of experience, Kymos Group provides the following services:
Bioanalysis
Bioanalysis Experts: Precision and knowledge
Kymos Group stands at the forefront of bioanalysis, providing precise and reliable testing solutions for every phase of drug development. With over 20 years of expertise, we specialize in bioanalysis for small molecules, biologics, and nucleic acids, ensuring compliance with the highest industry standards. Our cutting-edge technologies and deep scientific knowledge enable us to support our clients and provide high-quality and tailor-made services oriented to reduce their time-to-market:
Events
Join us at our upcoming events
Making Pharma Spain: Barcelona, ES | 18-19 Feb 2026
PharmaForum: Mainz, DE | 05 Mar 2026
KOREA PHARM & BIO: Seoul, KR | 31 Mar – 03 Apr 2026
Cell & Gene Meeting on the Med: Rome, IT | 28-30 Apr 2026
BIO KOREA 2026: Seoul, KR | 28-30 Apr 2026
Swiss Biotech Days: Basel, CH | 04-05 May 2026
Partnering Day Mabdesign: Lyon, FR | 12 May 2026
ICGT Congress: Gentilly, FR | 27-28 May 2026
Simposio AFI: Rimini, IT | 10-12 Jun 2026
Antibody Industrial Symposium: Montpellier, FR | 30 Jun – 01 Jul 2026
BIO Asia-Taiwan Exhibition: Taipei, TW | 16-19 Jul 2026
CPHI 2026: Milan, IT | 06-08 Oct 2026
Get in touch with us