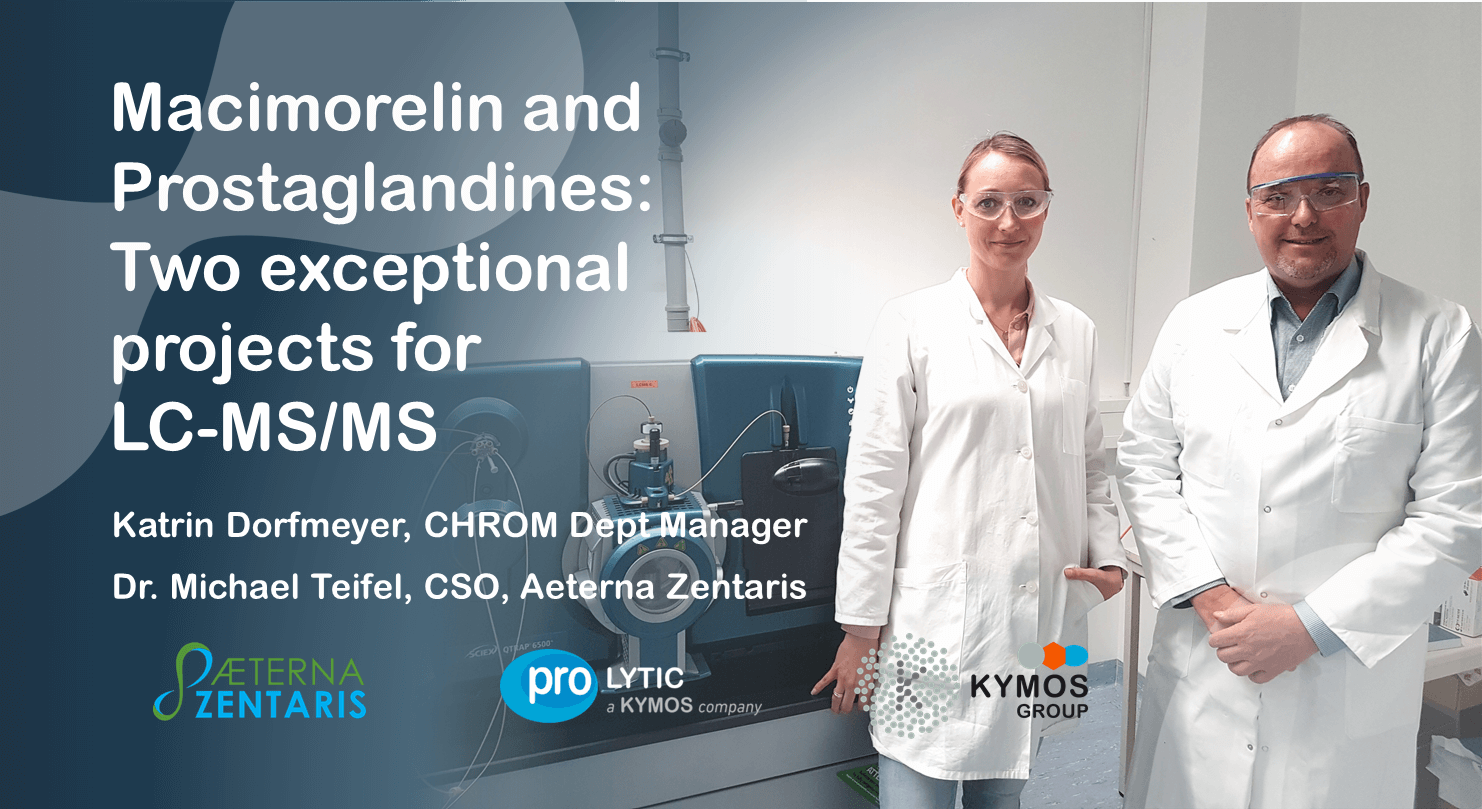
Kymos Christmas Calendar
This year the whole team of Kymos has a Christmas surprise for our clients and partners: An Online Christmas Calendar (Click here) Click on the link and find the Christmas greetings from our individual departments. Open a door every day, see funny and contemplative moments and find a surprise or two. We all had a (...) Read more