About Kymos Group
Kymos Group is a GLP/GMP-certified, GCP compliant, EMA, ANVISA, and FDA-inspected European contract research organization (CRO) offering an all-encompassing range of bioanalytical and quality control services for small molecules, biologics and advanced therapies.
The core of our being
Our Vision & Mission
We are fully committed to having a social impact by promoting diversity, gender equality, employee well-being, environmental protection, and social responsibility.
Our Vision is to be a world-class CRO & CLO offering a comprehensive and innovative portfolio of added value services contributing to the commercialization of pharmaceutical products for the ultimate benefit of patients.
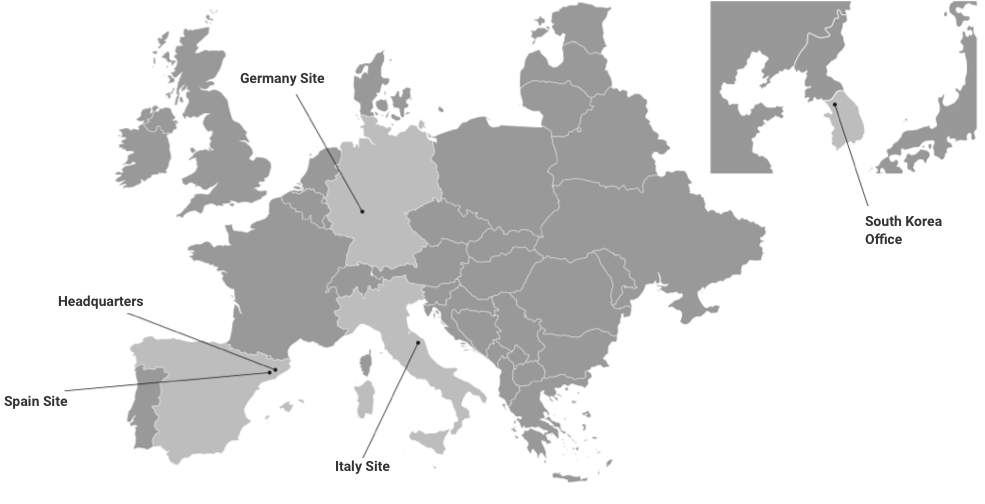
Three sites in Europe – One strong group
Our Global Reach
Our headquarters and main laboratory are in Barcelona (Kymos Spain), and additional laboratories are located in Frankfurt (Kymos Germany, originally Prolytic) and Ancona (Kymos Italy, originally Pharmaprogress).
Kymos Group is committed to supporting pharma, biotech, generic, and biosimilar clients in over 60 countries worldwide, helping them develop their products, reducing costs, and improving time to market with our unique, customized approach and multidisciplinary understanding of the entire development and manufacturing process: from early R&D, product development, preclinical, and clinical phases to final approval and post-marketing requirements.
Kymos Spain
Kymos S.L. was founded in Barcelona in 2001 by a team of R&D scientists with decades of experience in pharmaceutical development from early research to final formulation and scale up trials for commercialization.
- Bioanalysis of Small Molecules (aka Mass Spec and Permeation)
- Bioanalysis of Large Molecules (aka Immunology)
- Analytical Development and Validation
- Quality Control of Small Molecules
- Quality Control of Large Molecules (aka Biopharmaceutical testing)
- Elemental Analysis
- Microbiology
- Stabilities
Kymos Germany
Prolytic GmbH was founded in 2002 by a veteran group of scientists from Viatris GmbH, the largest business unit of Degussa/ASTA Medica AG. The newly formed CRO, based in Frankfurt, focused on the delivery of bioanalytical and pharmacokinetics services to clients in the pharmaceutical sector. In 2020 Prolytic became part of the Kymos group.
- Bioanalysis of Small Molecules (aka Chromatography)
- Bioanalysis of Large Molecules (aka Biology)
- Bioanalysis of Nucleic Acids
Kymos Italy
Pharmaprogress s.r.l. was legally founded in 1999 by former Angelini group executive and began operating as a CRO in 2001. A 700m2 fit-to-purpose laboratory was built in Camerata Picena, near Ancona, and was GMP approved by Italian Health Authorities.
Later in 2023, the facilities were moved to Monsano where a new laboratory of 1800m2 was built and inaugurated the following year.
- Stabilities, Analytical Development and Validation
- Quality Control of Small Molecules (aka Quality & Mass Spectrometry)
Kymos Branch in South Korea
In 2024, Kymos Group established a Liaison Office in Seoul, South Korea, to enhance support for our international clients across the Asia-Pacific (APAC) region.
Located in the prestigious ASEM Tower, this office serves as our primary contact point for pharmaceutical and biotechnology companies operating in the region.
ABOUT US
Our Commitment & Success
Rather than just a service provider, Kymos aims to be a reliable partner: Fully committed to our clients’ goals and the projects we are entrusted with; delivering high-quality services, reliable in terms of pricing in order to sustain our client’s competitiveness; upholding deadlines because the time to market is a key element to success; and constantly dedicated to research and development as the only way to meet the new challenges of a rapidly-changing business environment.
We are constantly growing at 2-digit percentages thanks to the implementation of new services and capabilities.
We are proud to have a very high percentage of returning clients on a yearly basis.
We have a high ratio of quotations approved, reflecting a consistent offer in terms of quality and fair prices.
The percentage of new clients is increasing year after year, mainly due to our efforts in internationalization.
From Vision to growth
Our History
Kymos S.L. was founded in 2001 by a team of R&D scientists with decades of experience in pharmaceutical development from early research to final formulation and scale up trials for commercialization. The company was established at the Barcelona Science Park and rapidly expanded despite high competition. In 2011 Kymos took over Ipsen Pharma’s immunology team to create a pioneering biological division. In 2015 Kymos moved to a brand-new laboratory at the Vallès Technology Park. In 2016 Kymos acquired Italian CRO Pharmaprogress. In 2018, steady growth called for two successive extensions with a new office building and laboratories. In 2020 Kymos acquired German CRO Proytic. In 2021 the Kymos Group is made up of a two-building HQ plus two European sites, 14 laboratory departments, 170 highly trained experts, and 5,000 m2 worth of facilities and top-of-the-line instruments. In 2023-2024 our Italian site was expanded with a new building, and a lab extension has started in Spain.

Pharmaprogress begun to provide services to third parties as CRO in 2001, despite the legal entity was previously founded in 1999. A fit-to-purpose laboratory of 700 m2 was built in Camerata Picena, near Ancona and it was GLP approved by Italian Health Authorities. The Company was founded by a former manager of an Italian pharmaceutical company.

Prolytic was founded. The roots of Prolytic GmbH can be traced back to Viatris GmbH & Co. KG, part of ASTA Medica AG, a pharmaceutical research company and formerly wholly owned subsidiary of Degussa AG. Viatris GmbH & Co. KG was sold to a finance investor who closed down the drug development sections “Bioanalysis” and “Pharmacokinetics.” An experienced group of scientists from these sections founded Prolytic GmbH in December 2002 with the aim of offering their abundant experience in bioanalysis and pharmacokinetics to clients in the pharmaceutical sector.

Kymos obtained the GLP certification in 2002, at the very beginning. The first projects were in metabolism and bioanalysis.

Prolytic was GLP certified by the Regierungspräsidium of Darmstadt in catergory 1 and 8.
Category 1: Physical and chemical properties and determination of content
Category 8: Analytical studies on biological materials

Pharmaprogress was GMP approved for chemical and physical testing by Italian Agency of Medicinal Products (AIFA) on January 2009.
The last authorization is n. aM-119/2016 of July 22nd, 2016, relating the following tests on Human Medicinal Products:
1.6.3 GMP Quality Control chemical and physical Tests
1.6.3 GMP Quality Control chemical and physical Tests on Investigational Medicinal Products

In 2009, the company obtained GMP and Manufacturer authorization (partial manufacturer for quality control activities). From this point, Kymos moved all CMC activities (mainly analytical development and stability studies) to GMP quality system and begun to provide quality control services for APIs and DPs.

In 2011, Kymos built a microbiology laboratory and extended its GMP certification to microbiological testing of non-sterile products. This certification was later extended further to cover sterile products in 2016.

In January 2012, Kymos reached an agreement to take over the immunology department of a former R&D centre that French multinational group IPSEN had in Barcelona. Thanks to this deal, Kymos was able to create a new biological division. Experienced staff, instruments and know-how were transferred to Kymos and both companies signed a long-term collaboration agreement to develop innovative drugs jointly.

In January 2015, the company moved its facilities from Barcelona (Barcelona Science Park) to a free-standing buiding in Cerdanyola del Vallès (Vallès Technology Park). A brand-new laboratory was built, increasing the surface area from 650 m2 to 1,500 m2. This meant the company could boost turnover and grow its staff in 2015 and 2016. The same year Kymos obtained the importation authorization for medicinal products. Batch release services for marketing products were implemented.

In November 2016, the company acquired a CRO in Italy called Pharmaprogress. The company was located in Ancona and had 8 people on staff. This company had a 700 m2 of laboratories for bioanalysis and analytical development of APIs and DPs. Nowadays, the company has been fully integrated into Kymos and now has 16 people on staff.

In July, Kymos underwent a general site inspection by the FDA. The inspection was completed succesfully and the corresponding EIR letter was issued.

In July, Kymos expanded its facilities moving the offices to a new free-standing building close to the existing one. This new building has a training room, an archive, different meeting rooms and a cafeteria.

In August, Prolytic moved to a larger and state of the art location at Weismüllerstraße 45. Doubling the size the 15-strong team now had 800 m² of laboratory and office space to offer the best bioanalytics and pharmacokinetics for its clients.

On April the FDA finished the inspection of Pharmaprogrees site in Ancona confirming this facility as eligible for marketing applications in the US. It has been a documentary inspection based on the previous AIFA (Agenzie Italiana del Farmaco) inspection thanks to the new Mutual Recognition Agreement existing between EMA e FDA.

In May, Kymos expanded again its facilities with new Analytical Development and Biopharmaceutical Testing laboratories. During the year, batch testing services for biosimilars and Asian clients are driving the company growth.

In October 2020, Kymos merged with the German bioanalytical laboratory Prolytic GmbH located in Frankfurt. Prolytic had 16 people on staff and was specialisted in bioanalysis of small, large molecules as well as oligo nucleotides and pharmacokinetics.

Kymos is a very international company. Besides the three sites in different European countries, we also work for clients all over the world. In 2021 we exceeded for the first time the number of 40 countries in which our customers are located covering all continents.
2022 Kymos acquired a more spacious, convenient, and state-of-the-art building in the Ancona area. Parts of the building need renovation and there is still a lot of work ahead of us. But next year, Pharmaprogress can finally move into its new modern facilities.

Kymos received the official certification by ANVISA (Agència Nacional de Vigilància Sanitária)for bioequivalence studies and was granted permission to participate in these studies for submission in Brazil. Work on Pharmaprogress new building has been finalized and we have started moving.

Our new building (1800 sqm) in Italy has been officially inaugurated at the start of the year, and all the equipment and projects have been moved to our new location in Monsano. Work has started on the lab extension in Spain (+600 sqm).

Get in touch with us