
The European Medicines Agency (EMA) announced in October 2024 the adoption of the long-awaited guideline on the Quality and Equivalence of Topical Products. The final document was drafted back in 2018 and will be coming into effect in April 2025 with the official title “Guideline on quality and equivalence of locally applied, locally acting cutaneous products”.
Our IVRT & IVPT and Quality Assurance departments have summarized the most relevant aspects of the EMA guideline with regard to IVRT and IVPT, dividing them between the quality recommendations and the therapeutic equivalence section. Finally, they have shared Kymos Group’s experience working in compliance with it since its previous draft document in 2018.
What is the new EMA’s Guideline on Topical Products for 2024?
As the title suggests, this guideline relates to locally applied and locally acting medicinal products for cutaneous use but may also be relevant for other products such as preparations for auricular or ocular use, and locally acting vaginal products. This new regulation is divided into quality and equivalence recommendations and introduces a more structured approach to evaluating cutaneous products.
The second part regarding equivalence does not apply to biological medicinal products, herbal medicinal products, products where equivalence with respect to efficacy is demonstrated by clinical trials, and products where the pharmaceutical form of the test and reference are not the same. This exclusion is especially important when dealing with systemic absorption dosage forms such as transdermal patches.
With these recent updates, EMA emphasizes a more structured and stepwise approach that should simplify the process of demonstrating therapeutic equivalence focusing on in vitro (In Vitro Release Test or IVRT, and In Vitro Permeation Test or IVPT) and pharmacokinetic (PK) methods that present cost-saving and time-efficient alternatives to clinical studies.
Quality Recommendations for Topical Products
The quality recommendations from the guidance apply to new marketing authorization applications and post-approval changes for products that are not covered by other guidelines or the relevant Pharmacopoeia standards. Concerning IVRT and IVPT the most relevant aspects of the guideline are:
Pharmaceutical Development
-
Formulation Development
The formulation development should be aligned with the QTTP (Quality Target Product Profile), with suitable tests to characterize and control CQAs (Critical Quality Attributes) such as ease of administration, duration of use, and product performance like dissolution, IVRT and if appropriate IVPT.
-
Product Characterization
Characterization is necessary to facilitate life-cycle management, and if necessary, product equivalence. Key performance tests should include dissolution of suspensions, IVRT, and IVPT if necessary. Product performance should be shown to be stable during storage.
Control Strategy
CQAs crucial for drug release control should be carefully managed, with tests such as IVRT and IVPT (if relevant). Other parameters (e.g. microscopy, DSC, rheology) may be used if they are proven to be more discriminative when controlling drug release. Additionally, any performance test limits (e.g., dissolution, IVRT) included in the specification must be justified by reference to clinical batches with proven efficacy and safety.
Stability Program
Stability testing must ensure the product’s quality and effectiveness over time, with IVRT or other performance tests confirming shelf-life consistency.
Equivalence Recommendations for Topical Products
This part of the guidance applies to new cutaneous products that want to demonstrate therapeutic equivalence with an existing medicinal product. It is also applicable to post-approval changes when a potential impact on quality, safety, or efficacy is expected after a risk assessment.
The guide also specifically states that “in the case of applications which rely on literature to demonstrate the safety and efficacy of the medicinal product, the relevance of the literature should be supported by equivalence bridging data to the product described in the literature”.
As stated before, the EMA recommends a stepwise approach for demonstrating equivalence. This allows pharmaceutical manufacturers to know beforehand which tests to perform for their products (simple formulations such as solutions or gels, or complex formulations like emulsions), and its primary goal is to skip full clinical studies.
When deciding which approach and decision tree to select, the EMA takes into consideration the qualitative composition, the quantitative composition, and the physicochemical and structural characterization of the cutaneous products:
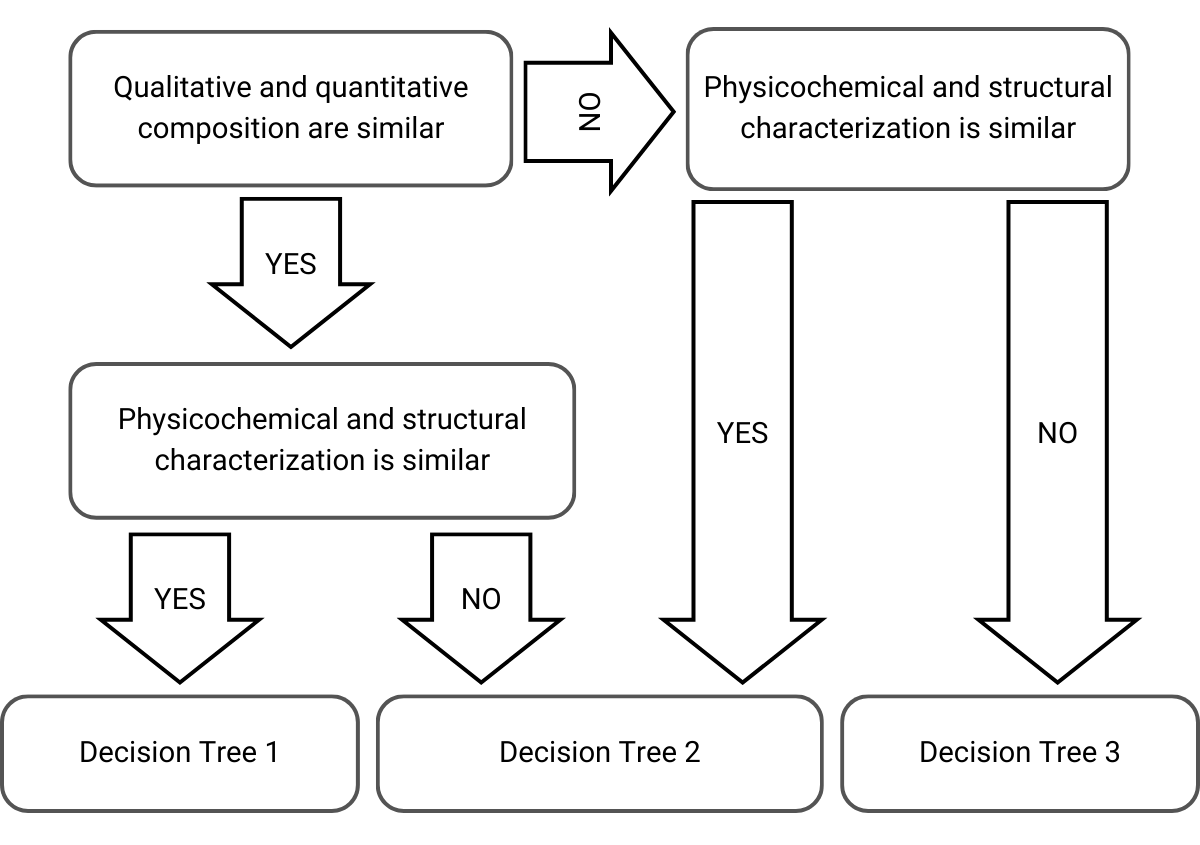
Figure 1) Selection of decision tree in the stepwise approach adapted from EMA’s guideline
Then, depending on these considerations, the stepwise approach is as follows on the next decision trees leading into acceptable bridges or rejections:
Decision Tree 1: Same qualitative and quantitative composition, and same physicochemical and structural characterization
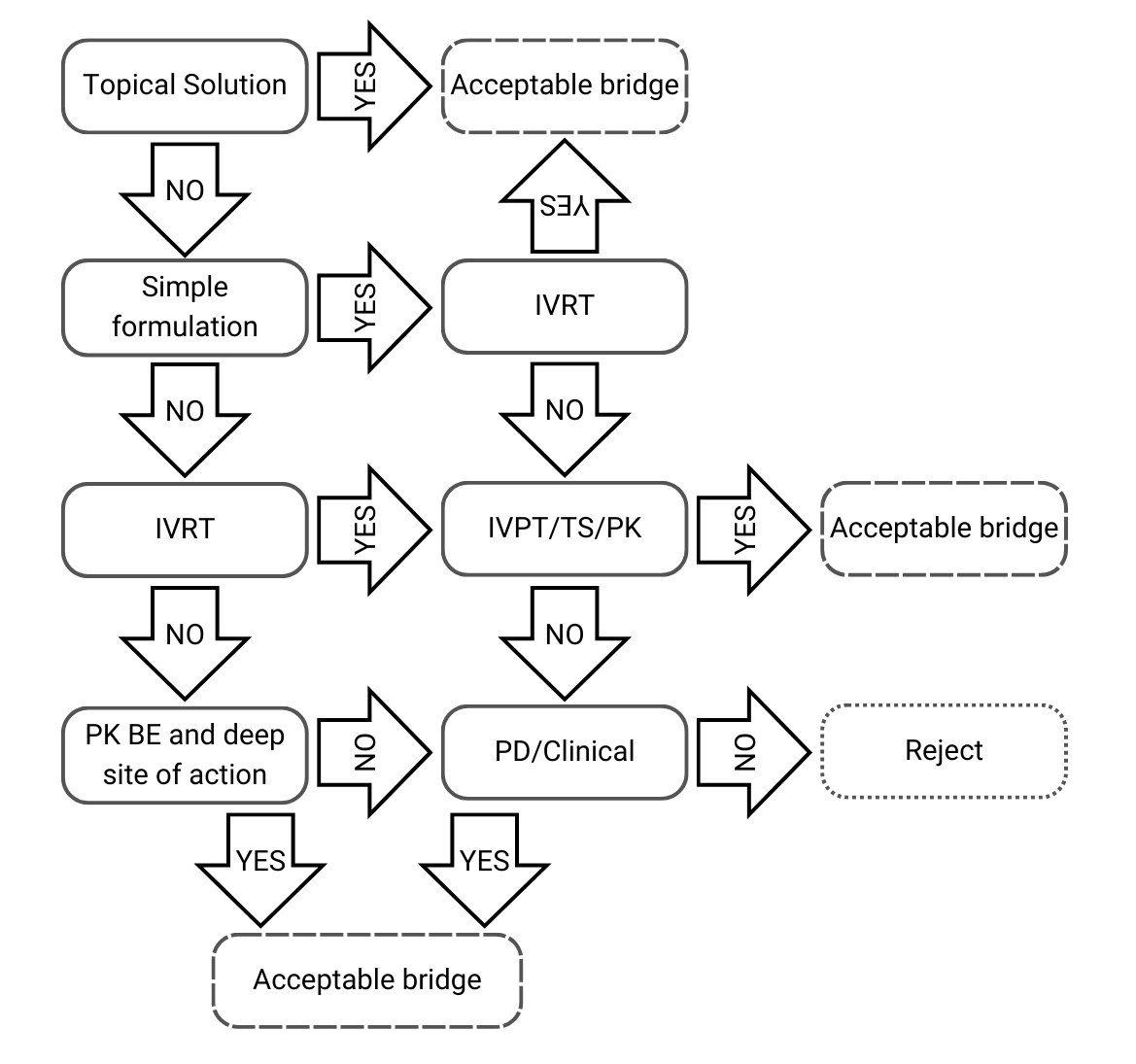
Figure 2) Decision Tree 1 from EMA’s guideline
Decision Tree 2: Small differences in qualitative and quantitative compositions, and/or physicochemical and structural characterization
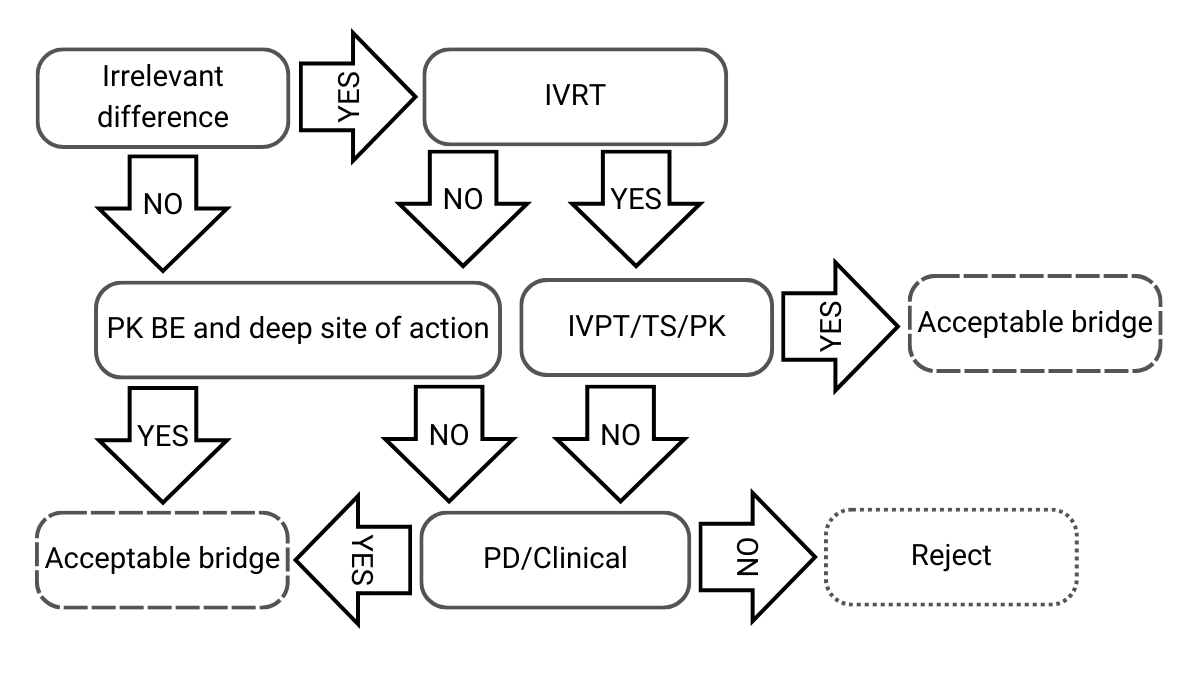
Figure 3) Decision Tree 2 from EMA’s guideline
Decision Tree 3: Different qualitative and quantitative compositions and different physicochemical and structural characterization
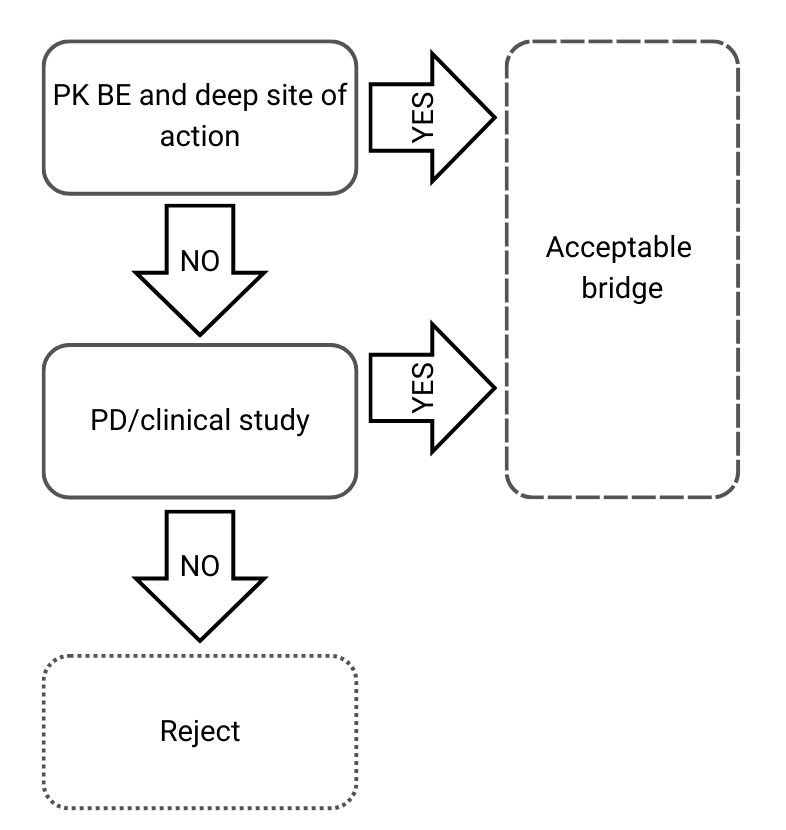
Figure 4) Decision Tree 3 from EMA’s guideline
As seen in these decision trees, depending on the similarities between the products, the EMA offers different stepwise approaches that manufacturers should consider.
Kymos Group’s experience with IVRT and IVPT studies
Kymos Group has a specialized team working with drug release and techniques for topical products since 2017 and has extensive experience with IVRT and IVPT studies. Our methods have been developed in compliance with the draft version of this same guideline from 2018, and our scientists are already familiar with this final version.
We’ve divided our catalog of drug release and percutaneous absorption services into two main groups:
- IVRT: We measure drug release amounts and rates using artificial membranes to develop and validate methods for different formulations. These methods can be used in comparative studies to evaluate equivalence and also to perform the quality control of manufacturing batches.
- IVPT: We measure transdermal permeated amounts, flux rates, and layer distribution with skin samples for bioequivalence studies. We can also aid in the optimization and comparison of formulations with percutaneous absorption studies.
Our laboratory is one of the few European facilities offering both GLP- and GMP-certified percutaneous absorption assays for cutaneous products with the latest automated Hanson vertical diffusion Phoenix instruments (Franz cells testing).
With a deep understanding of the new guidelines, our scientific team is ready to support clients from formulation development to regulatory submission of new marketing applications, but also for cutaneous products that want to demonstrate equivalence with existing medicinal products following the stepwise approach from the EMA.
Conclusions
The adoption of the new EMA guideline on the quality and equivalence of topical products is a big advancement towards a more harmonized but also more structured approach to the analysis of these types of medicinal products. Its stepwise recommendations for demonstrating equivalence using techniques such as IVRT and IVPT streamline the process of bringing new generics to market without the need for costly and time-consuming clinical studies.
As a leading CRO in IVRT and IVPT, Kymos Group and its team are ready to support clients and partners at each step, ensuring a smooth path to market approval in Europe under the latest guidelines.
If you need more information about the EMA guideline or any assistance with your percutaneous absorption projects, please contact commercial@kymos.com. We will be pleased to provide you with detailed consulting and support.

